Within the box are 3 more compact bins positioned horizontally having an arrow top from the primary to the next and from the next for the 3rd. These bins are meant to depict The three phases of your cleaning validation program.
Permissible each day publicity – The PDE represents a material-particular dose that may be not likely to lead to an adverse result if an individual is uncovered at or underneath this dose on a daily basis for the lifetime. (
In the last number of a long time, different cleaning validation assistance paperwork have presented the sector with insight on how to comply with personal nation regulations.2
Defining acceptance requirements remains perhaps the most tough element of a cleaning validation method.
A] Keeping style: This method shall be adopted, by means of equipment design and style; it is feasible to retain the rinse volume.
Transfer the swab applying gloved worn hand in to the take a look at tube and analyze it as per the validated analytical method.
The HBEL of the new products and Appraise the suitability of your product on your facility and whether or not committed services/gear or other extra controls are expected.
QRM principles must be Employed in environment appropriate limitations for have more than making an allowance for the production system as well as the stage of manufacture. Stringency of restrictions might increase throughout the purification system.
machines need to be built in accordance Along with the identical ideas as used for concluded drug products and solutions
,fifteen Manufacturers may possibly would like to evaluate and Review different ways to residue boundaries calculation to decide which most here closely fits cGMP needs, company procedures, and internet site goals.
A similar course of action shall be relevant for that individual product throughout regimen cleaning routines following the productive completion of cleaning validation.
Particular challenge trials may very well be required. The goal is always to identify important cleaning parameters and fully grasp the influence of variability of these parameters on cleaning overall performance.
You should have self-confidence that cleaning processes might be helpful and reproducible in comprehensive/professional scale machines. Cleaning verification(s) might be carried out in complete-scale gear as the last stage of cleaning get more info procedure style and development to confirm understanding of the effectiveness of the proposed cleaning process.
This template is employed to finish the procedure validation protocol by reporting the verification in the products/procedure remaining style towards the consumer, useful, and/or style and design technical specs. Conveniently determine important gear components, utilities supply, and environmental requirements.
 Michelle Pfeiffer Then & Now!
Michelle Pfeiffer Then & Now! Destiny’s Child Then & Now!
Destiny’s Child Then & Now! Kenan Thompson Then & Now!
Kenan Thompson Then & Now!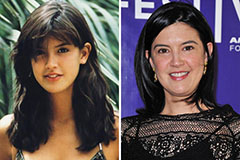 Phoebe Cates Then & Now!
Phoebe Cates Then & Now!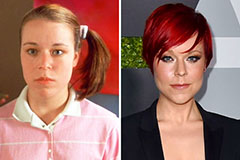 Tina Majorino Then & Now!
Tina Majorino Then & Now!